primipil/iStock via Getty Images
In individuals, insanity is rare; but in groups, parties, nations and epochs, it is the rule.”― Friedrich Nietzsche
Today, we take an in-depth look at a small biotech concern that is based overseas. Its unique business model, pipeline, and recent insider buying merited further investigation. An analysis follows below.
Seeking Alpha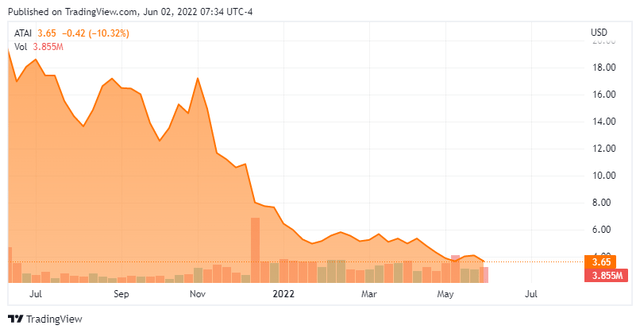
Company Overview:
Atai Life Sciences N.V. (NASDAQ:ATAI) is a Berlin, Germany-headquartered clinical-stage biopharmaceutical concern focused on developing therapies for the treatment of mental health disorders. The company operates under a decentralized model that either acquires a controlling (or significant) interest in treatment programs or creates wholly-owned subsidiaries de novo. Atai was founded in 2018 and went public in June 2021, raising net proceeds of $231.6 million at $15 per share. Its stock trades around $3.75 a share, translating to a market cap of approximately $650 million.
March Company Presentation
Through its variable interest entities and wholly-owned subsidiaries in the U.S., Australia, UK, Canada, and Germany, the company has constructed a pipeline of 11 programs and six enabling technologies. Each are led by focused teams with Atai providing investment, as well as the developmental and operational infrastructure. The company’s criteria for evaluating potential program candidates include prior evidence of efficacy in humans, rapid proof-of-concept (i.e., modestly sized, short duration clinical trials), blockbuster peak-sales potential, and synergies with its existing pipeline. By altering the administration route or half-life of a compound with previous human trials backing its potential effectiveness, Atai seeks to move metal health assets quickly through the clinic. Through its investments, Atai currently has an interest in six clinical programs.
March Company Presentation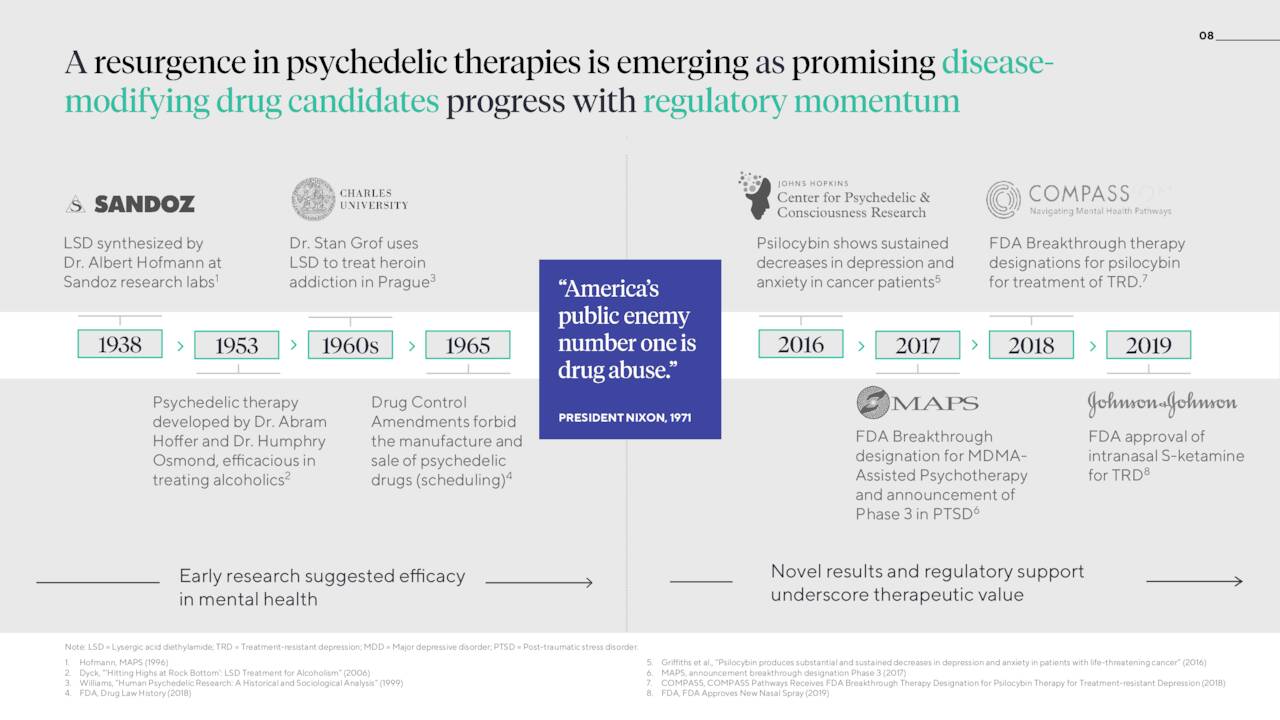
One of the company’s key areas of concentration is psychedelics, which despite previous stigmas surrounding their usage recreationally, have potentially significant therapeutic benefit for the treatment of depression, substance use disorders, and other mental health conditions.
Clinical Assets
March Company Presentation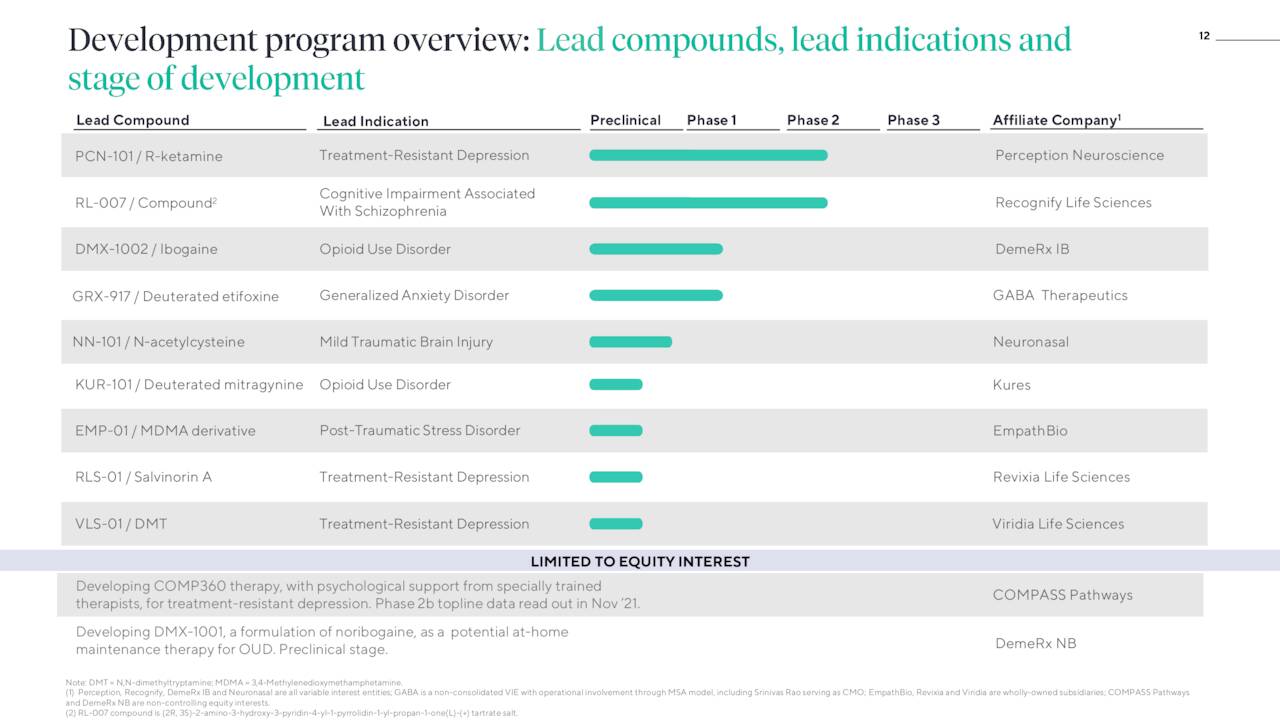
PCN-101. One of the Atai’s more advanced programs is PCN-101, a parenteral (in this instance intravenous and ultimately subcutaneous) formulation of R-ketamine (arketamine), a glutamatergic modulator that is a component of racemic ketamine, which is being investigated as a rapid-acting remedy for treatment resistant depression (TRD). At therapeutic doses, it is thought to be non-psychedelic and has the potential to be an alternative to Johnson & Johnson’s (JNJ) psychedelic S-ketamine (esketamine) nasal spray marketed as Spravato, which is burdened with dissociative side effects. Another formulation of R-ketamine produced rapid and durable responses with a four-fold lower affinity for the NMDA receptor – thought to be connected to the dissociative…
Read more:Atai Life Sciences N.V.: A First Take (NASDAQ:ATAI)









 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  Solana
Solana  Dogecoin
Dogecoin  Cardano
Cardano  USDC
USDC  Lido Staked Ether
Lido Staked Ether  Shiba Inu
Shiba Inu  Avalanche
Avalanche  TRON
TRON  Toncoin
Toncoin  Stellar
Stellar  Wrapped stETH
Wrapped stETH  Polkadot
Polkadot  Wrapped Bitcoin
Wrapped Bitcoin  Chainlink
Chainlink  WETH
WETH  Bitcoin Cash
Bitcoin Cash  Sui
Sui  Pepe
Pepe  Litecoin
Litecoin  NEAR Protocol
NEAR Protocol  Hedera
Hedera  LEO Token
LEO Token  Uniswap
Uniswap  Wrapped eETH
Wrapped eETH  Aptos
Aptos  Internet Computer
Internet Computer  USDS
USDS  Cronos
Cronos  Ethereum Classic
Ethereum Classic  POL (ex-MATIC)
POL (ex-MATIC)  Artificial Superintelligence Alliance
Artificial Superintelligence Alliance  Bittensor
Bittensor  Render
Render  Ethena USDe
Ethena USDe  Filecoin
Filecoin  Algorand
Algorand  Arbitrum
Arbitrum  Stacks
Stacks  Dai
Dai  Celestia
Celestia  Bonk
Bonk  Cosmos Hub
Cosmos Hub  Immutable
Immutable